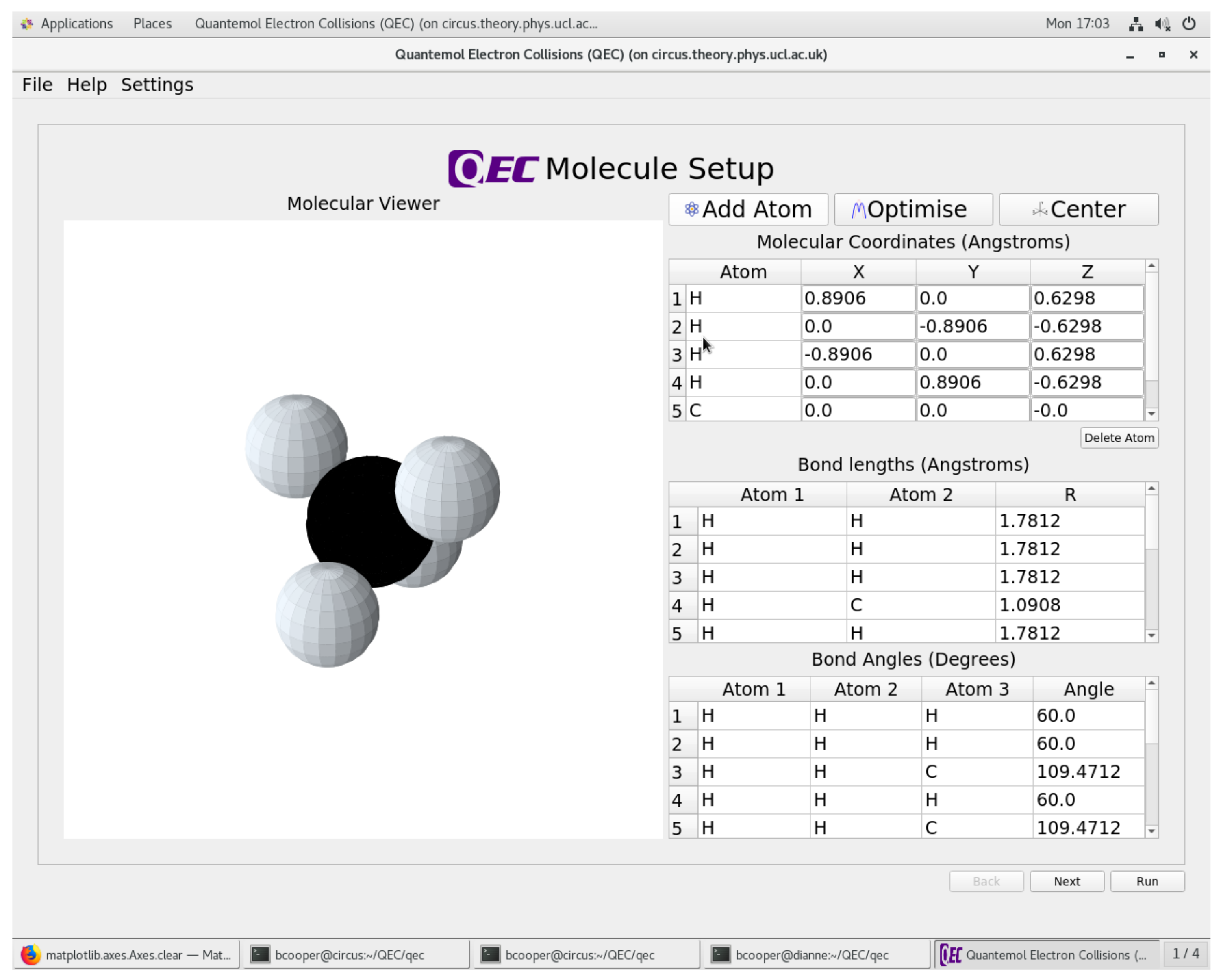
Atoms | Free Full-Text | Quantemol Electron Collisions (QEC): An Enhanced Expert System for Performing Electron Molecule Collision Calculations Using the R-Matrix Method
What happens when a photon collides with an electron of a different energy level Does it deflect? Go through it? Magically prevented from interacting? - Quora

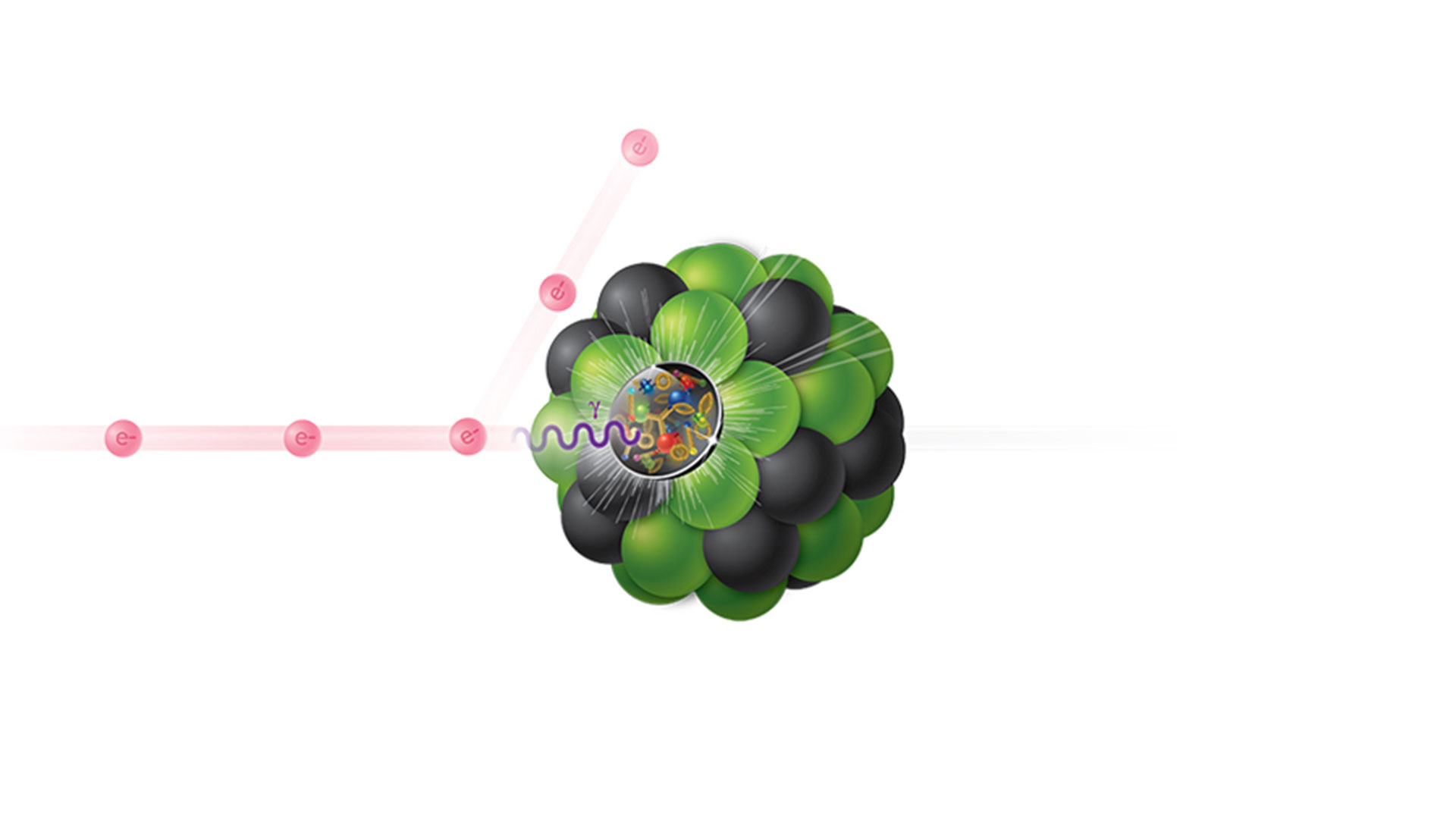
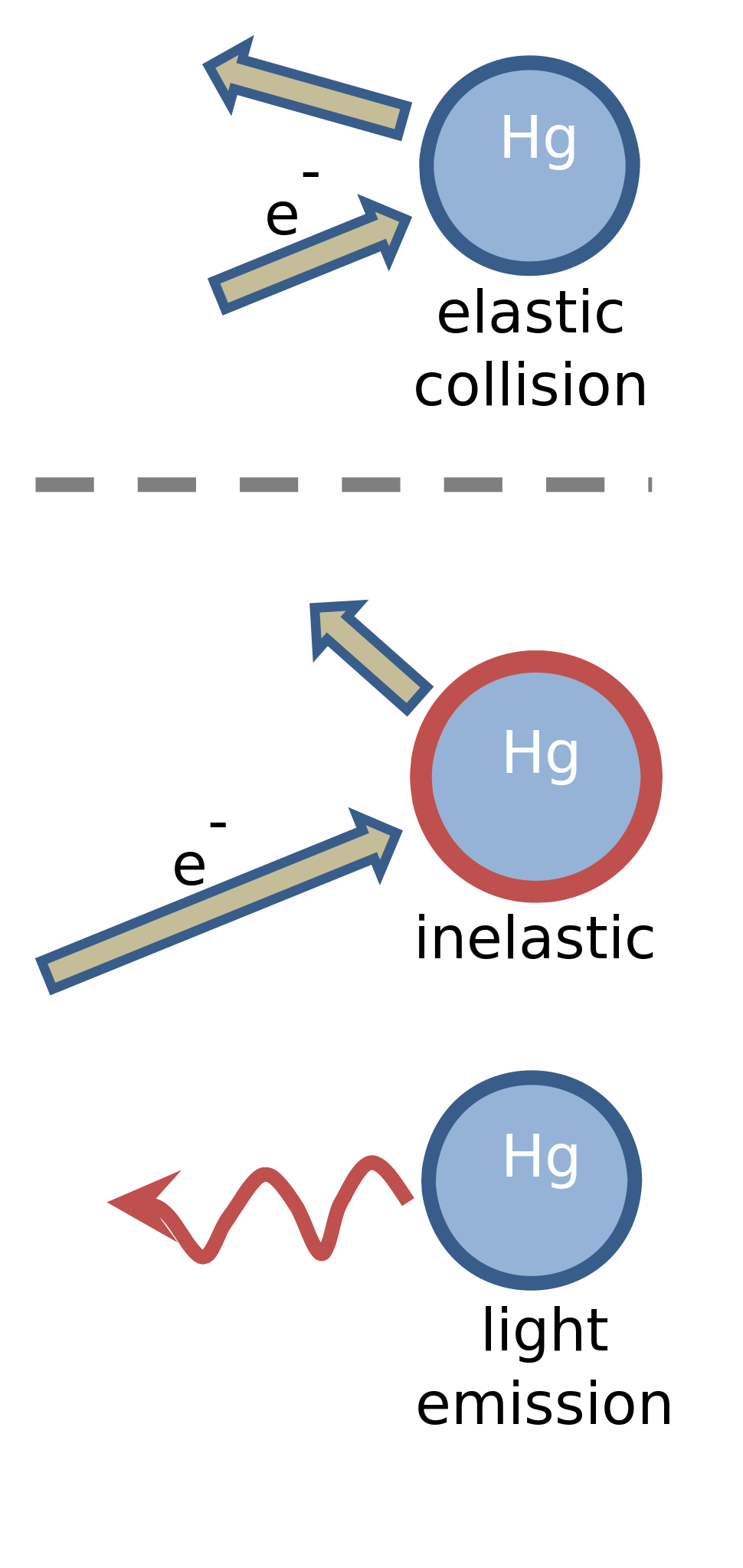
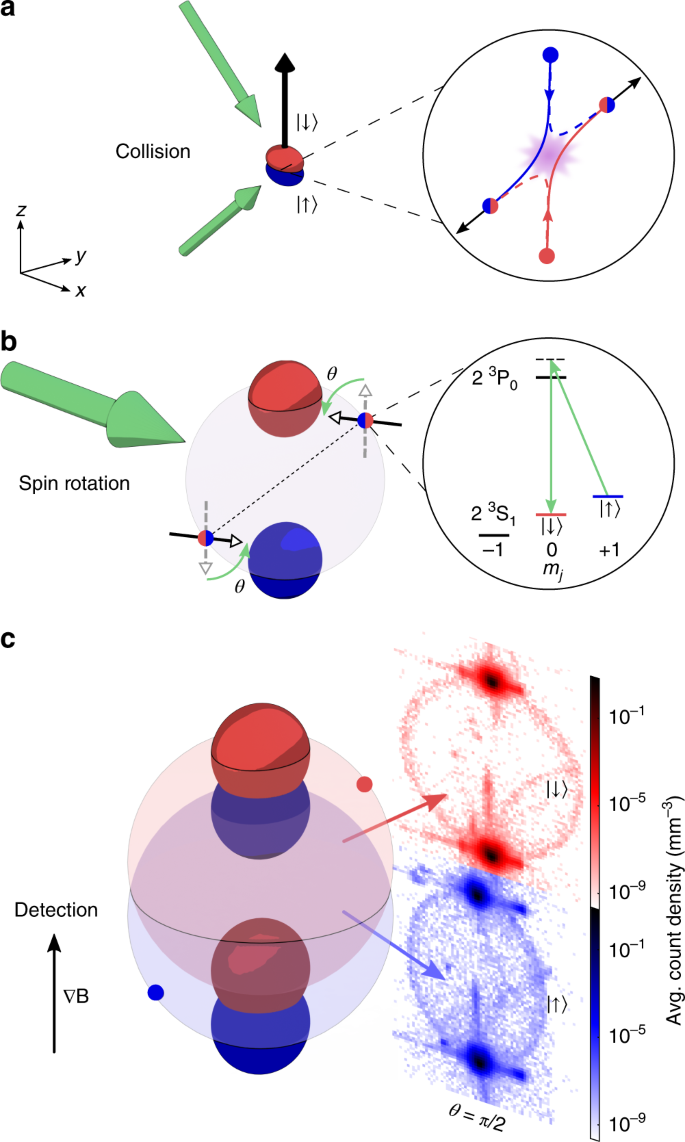
Electron collisions with atoms, ions, molecules, and surfaces: Fundamental science empowering advances in technology | PNAS
Electron collisions with atoms, ions, molecules, and surfaces: Fundamental science empowering advances in technology

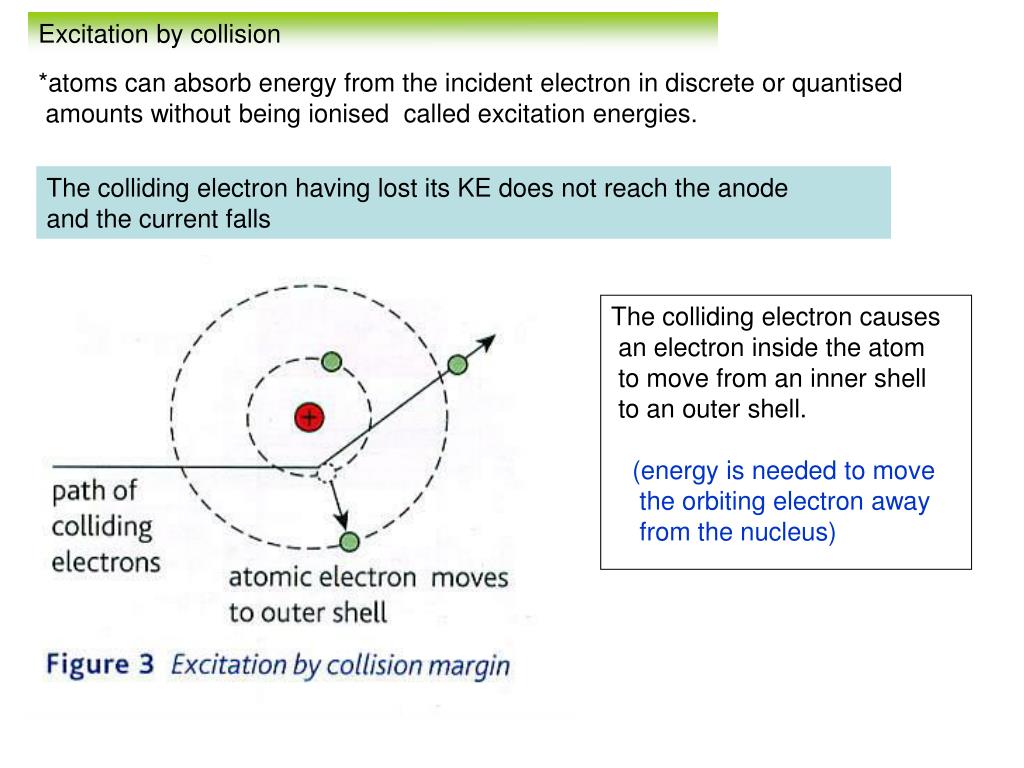
When high energy electrons in a discharge tube collide with the anode, penetrating radiations are produced which are named X-rays. Why are these radiations produced when electrons collide with the anode? -

11. Parameters in an electron collision with atom (a is the classical... | Download Scientific Diagram

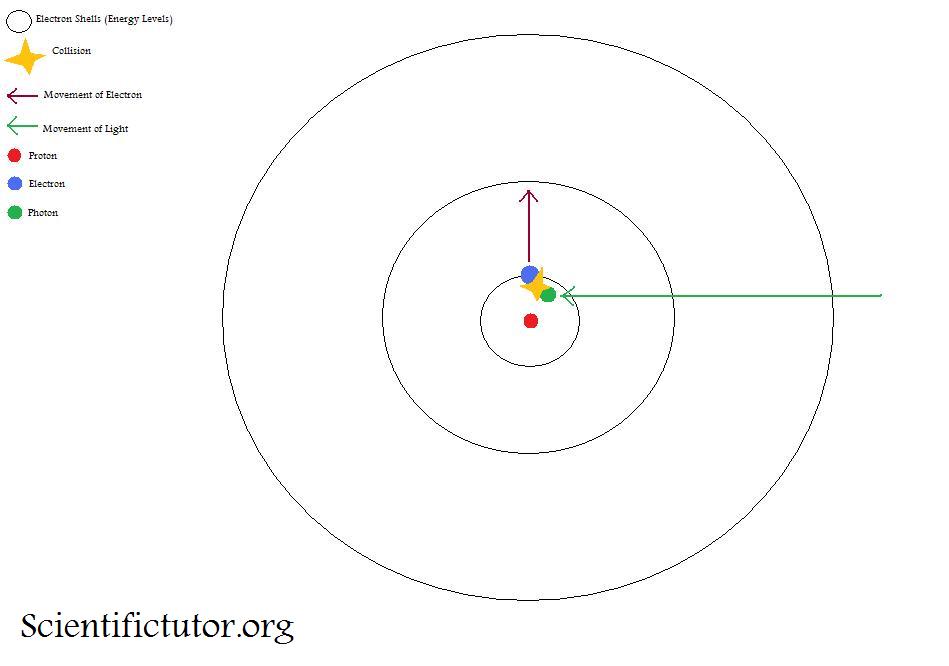
electricity - What do we mean when we say an electron collides with a molecule or atom? - Physics Stack Exchange

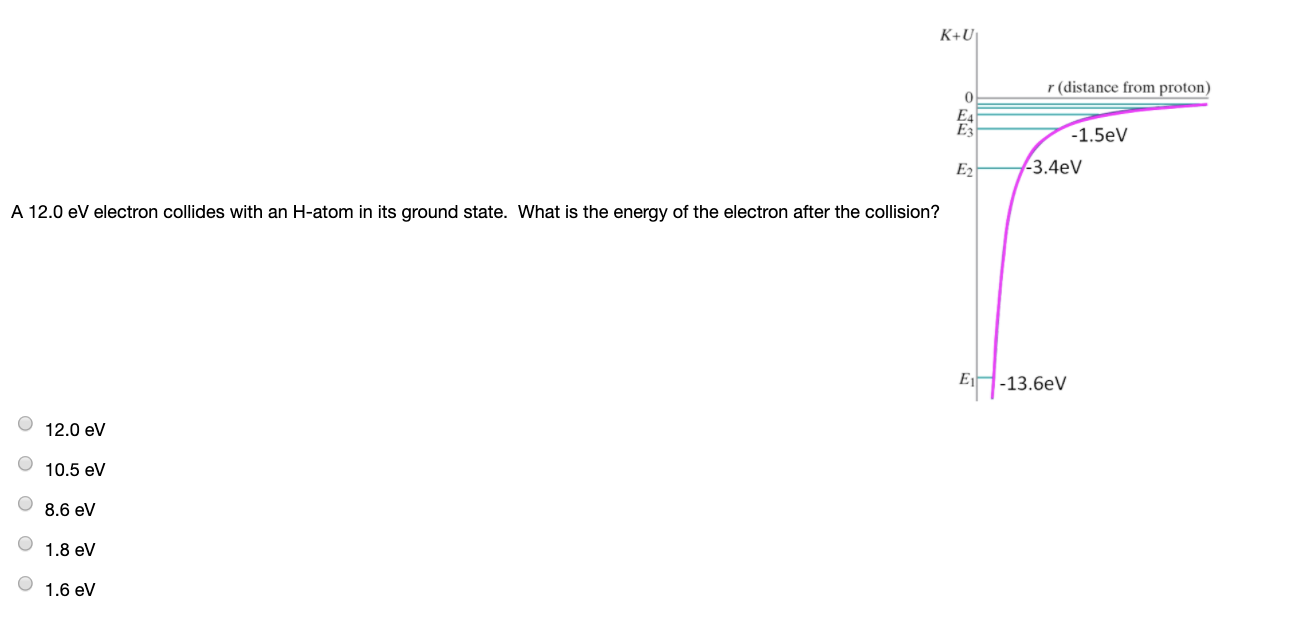
SOLVED:An electron collides elastically with a stationary hydrogen atom. The mass of the hydrogen atom is 1837 times that of the electron. Assume that all motion, before and after the collision, occurs